(Family Features) When kidney cancer spreads or becomes advanced, it can be challenging to treat. Common signs and symptoms can include blood in urine, lower back pain on one side, a mass on the side or lower back, loss of appetite or unexplained weight loss.
While a diagnosis of advanced kidney cancer can be overwhelming, there are steps patients can take to feel more empowered as they face the disease, starting with learning about the treatments that may be available for them.
Exploring Your Options
When facing advanced kidney cancer, it’s important for patients and caregivers to have open communication with their healthcare team to understand the diagnosis and establish a treatment plan.
Asking questions to understand where the cancer has spread, what the expected prognosis is and the potential benefits of treatment – including the possibility to live longer – can be critical to aligning on a path forward.
Fortunately, there are several types of medicines available for advanced kidney cancer today, depending on the specifics of each patient’s disease. Chemotherapy, targeted therapy or immunotherapy are a few types of treatment that may be considered, sometimes in combination. Immunotherapy works differently than chemotherapy or targeted therapy, as it helps a person’s own immune system to fight cancer and can enable the immune system to find and attack cancer cells. For some patients, dual immunotherapy – or a combination of two immunotherapy treatments – may be recommended.
For example, Opdivo (nivolumab) + Yervoy (ipilimumab) is approved by the U.S. Food and Drug Administration (FDA) as a combination of two immunotherapies for certain newly diagnosed adults whose kidney cancer (also referred to as renal cell carcinoma) has spread. It is not known if Opdivo is safe and effective in children younger than 18 years of age. Opdivo (10 mg/mL) and Yervoy (5 mg/mL) are injections for intravenous use.
This combination of two immunotherapies has the potential to work with the immune system in different but complementary ways to help fight cancer. While Yervoy may stimulate the kind of cells that help fight cancer, Opdivo may help these cells find and fight the cancer cells again.
While doing so, this immunotherapy combination can also affect healthy cells. These problems can sometimes become serious or life threatening and can lead to death. These problems may happen anytime during treatment or even after treatment has ended. You may have more than one of these problems at the same time. Some of these problems may happen more often when Opdivo is used in combination with Yervoy.
Opdivo and Yervoy can cause problems that can sometimes become serious or life-threatening and can lead to death. Serious side effects may include lung problems; intestinal problems; liver problems; hormone gland problems; kidney problems; skin problems; eye problems; problems in other organs and tissues; severe infusion reactions; and complications of stem cell transplant, including graft-versus-host disease (GVHD), that uses donor stem cells (allogeneic). Call or see your healthcare provider right away for any new or worsening signs or symptoms. Please see additional Important Safety Information below.
Understanding Overall Survival
One of the most important considerations for choosing a treatment is the potential for survival, or the chance to live longer. Overall survival is sometimes reported as a survival rate, which is the percentage of people in a clinical trial who are still alive for a certain time period after being diagnosed with or starting treatment for a disease, such as cancer.
“After my cancer diagnosis, my wife and I prayed about our future and pursuing every avenue with that goal in mind,” said Terry Broussard, who has been living with advanced kidney cancer. “I wanted a treatment that may give me a chance to live longer in order to see my youngest child graduate high school.”
Broussard’s doctor recommended treatment with Opdivo + Yervoy, which has overall survival data at five years. The FDA approval of this dual immunotherapy in advanced renal cell carcinoma (RCC) was based on results from the CheckMate -214 clinical trial, which included 847 previously untreated patients with kidney cancer that had spread and with one or more risk factors.
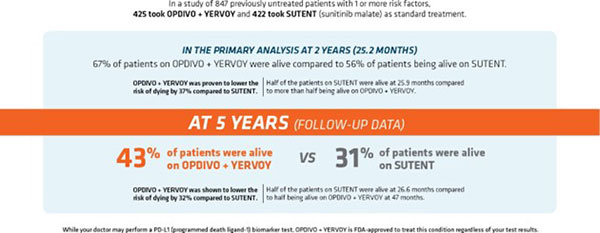
In the primary analysis at two years (25.2 months), the length of time patients lived without tumors worsening was 11.6 months for this immunotherapy combination and 8.4 months for sunitinib. There was no meaningful difference between the two treatments.
Researchers also assessed the overall response rate, which is a measure of the percentage of patients whose cancer shrunk (partial response) or disappeared completely (complete response) after treatment.
At the two-year time point, 41.6% of patients treated with Opdivo + Yervoy (95% CI:36.9-46.5) responded to treatment (n=177/425) versus 26.5% (n=112/422) of those treated with sunitinib (95% CI:22.4-31.0). Partial tumor shrinkage occurred in 32.2% of the patients treated with this immunotherapy combination compared to 25.4% of those treated with sunitinib. Tumors disappeared completely in 9.4% of patients treated with this immunotherapy combination versus in 1.2% of patients treated with sunitinib. The disappearance of any measurable tumors in response to treatment does not necessarily mean the cancer has been cured. Opdivo + Yervoy will not work for everyone. Individual results may vary.
“Advanced kidney cancer is a complex disease with many treatment options, which can feel overwhelming for people facing a devastating cancer diagnosis,” said Ulka Vaishampayan, M.D., professor, Internal Medicine, Division of Hematology/Oncology, University of Michigan. “The goal of treatment is to help patients live longer, and research like these five-year data gives us insight into what treatment with Opdivo + Yervoy may look like for patients from the trial over time.”
The most common side effects of Opdivo, when used in combination with Yervoy, include: feeling tired; diarrhea; rash; itching; nausea; pain in muscles, bones, and joints; fever; cough; decreased appetite; vomiting; stomach-area (abdominal) pain; shortness of breath; upper respiratory tract infection; headache; low thyroid hormone levels (hypothyroidism); constipation; decreased weight; and dizziness.
Establishing and Leaning on a Support System
From diagnosis to treatment and beyond, many patients find the support from family, friends and loved ones invaluable. Identifying a friend, spouse or caregiver who can join doctor appointments, ask questions and take notes can be a helpful way to track all the details that can often be overwhelming when facing cancer. “I’ve been incredibly lucky to have the support of my wife, children, nurses and doctors every step of the way,” said Broussard. “Even in the most challenging moments, knowing they were by my side gave me the hope and inspiration I needed to continue moving forward.”
To learn more, visit Opdivo.com.
Source: Bristol Myers Squibb
Photo caption: Terry Broussard and his wife, Tracy. Broussard is an actual patient who has been compensated by Bristol Myers Squibb for his time.
INDICATION AND IMPORTANT SAFETY INFORMATION
OPDIVO® (nivolumab) is a prescription medicine used in combination with YERVOY® (ipilimumab) to treat adults with kidney cancer in certain people when your cancer has spread (advanced renal cell carcinoma) and you have not already had treatment for your advanced RCC.
It is not known if OPDIVO is safe and effective in children younger than 18 years of age.
Information provided in this article is not a substitute for talking with your healthcare professional. Your healthcare professional is the best source of information about your disease.
Important Safety Information for OPDIVO® (nivolumab) + YERVOY® (ipilimumab)
What is the most important information I should know about OPDIVO + YERVOY?
OPDIVO and YERVOY are medicines that may treat certain cancers by working with your immune system. OPDIVO and YERVOY can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. These problems may happen anytime during treatment or even after your treatment has ended. You may have more than one of these problems at the same time. Some of these problems may happen more often when OPDIVO is used in combination with another therapy.
What are the serious side effects of OPDIVO + YERVOY?
Call or see your healthcare provider right away if you develop any new or worse signs or symptoms, including:
- Lung problems: new or worsening cough; shortness of breath; chest pain
- Intestinal problems: diarrhea (loose stools) or more frequent bowel movements than usual; stools that are black, tarry, sticky, or have blood or mucus; severe stomach-area (abdominal) pain or tenderness
- Liver problems: yellowing of your skin or the whites of your eyes; severe nausea or vomiting; pain on the right side of your stomach area (abdomen); dark urine (tea colored); bleeding or bruising more easily than normal
- Hormone gland problems: headaches that will not go away or unusual headaches; eye sensitivity to light; eye problems; rapid heart beat; increased sweating; extreme tiredness; weight gain or weight loss; feeling more hungry or thirsty than usual; urinating more often than usual; hair loss; feeling cold; constipation; your voice gets deeper; dizziness or fainting; changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
- Kidney problems: decrease in your amount of urine; blood in your urine; swelling in your ankles; loss of appetite
- Skin problems: rash; itching; skin blistering or peeling; painful sores or ulcers in the mouth or nose, throat, or genital area
- Eye problems: blurry vision, double vision, or other vision problems; eye pain or redness.
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with OPDIVO and YERVOY. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
- Chest pain; irregular heart beat; shortness of breath; swelling of ankles
- Confusion; sleepiness; memory problems; changes in mood or behavior; stiff neck; balance problems; tingling or numbness of the arms or legs
- Double vision; blurry vision; sensitivity to light; eye pain; changes in eye sight
- Persistent or severe muscle pain or weakness; muscle cramps
- Low red blood cells; bruising
Getting medical help right away may help keep these problems from becoming more serious. Your healthcare team will check you for these problems during treatment and may treat you with corticosteroid or hormone replacement medicines. Your healthcare team may also need to delay or completely stop your treatment if you have severe side effects.
Possible side effects of OPDIVO + YERVOY
OPDIVO and OPDIVO + YERVOY can cause serious side effects, including:
- See “What is the most important information I should know about OPDIVO + YERVOY?”
- Severe infusion reactions. Tell your healthcare team right away if you get these symptoms during an infusion of OPDIVO or YERVOY: chills or shaking; itching or rash; flushing; shortness of breath or wheezing; dizziness; feel like passing out; fever; back or neck pain
- Complications, including graft-versus-host disease (GVHD), of bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be severe and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with OPDIVO or YERVOY. Your healthcare provider will monitor you for these complications.
The most common side effects of OPDIVO, when used in combination with YERVOY, include: feeling tired; diarrhea; rash; itching; nausea; pain in muscles, bones, and joints; fever; cough; decreased appetite; vomiting; stomach-area (abdominal) pain; shortness of breath; upper respiratory tract infection; headache; low thyroid hormone levels (hypothyroidism); constipation; decreased weight; and dizziness.
These are not all the possible side effects. For more information, ask your healthcare provider or pharmacist. You are encouraged to report side effects of prescription drugs to the FDA. Call 1-800-FDA- 1088.
Before receiving OPDIVO or YERVOY, tell your healthcare provider about all of your medical conditions, including if you:
- have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus
- have received an organ transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have received radiation treatment to your chest area in the past and have received other medicines that are like OPDIVO
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- are pregnant or plan to become pregnant. OPDIVO and YERVOY can harm your unborn baby
- are breastfeeding or plan to breastfeed. It is not known if OPDIVO or YERVOY passes into your breast milk. Do not breastfeed during treatment with OPDIVO or YERVOY and for 5 months after the last dose of OPDIVO or YERVOY.
Females who are able to become pregnant:
Your healthcare provider should do a pregnancy test before you start receiving OPDIVO or YERVOY.
- You should use an effective method of birth control during your treatment and for at least 5 months after the last dose of OPDIVO or YERVOY. Talk to your healthcare provider about birth control methods that you can use during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with OPDIVO or YERVOY. You or your healthcare provider should contact Bristol-Myers Squibb at 1-844-593-7869 as soon as you become aware of a pregnancy.
Tell your healthcare provider about all the medicines you take, including prescription and over-the- counter medicines, vitamins, and herbal supplements.
Please see U.S. Full Prescribing Information and Medication Guide for OPDIVO and YERVOY.
© 2023 Bristol-Myers Squibb Company. All Rights Reserved.
OPDIVO® and YERVOY® are registered trademarks of Bristol-Myers Squibb Company.
7356-US-2200719 2/23
Source:







